- Cancer Care Team
Cancer Care Team
To deliver optimal patient outcomesProducts and Services
Cancer Type
Supplies & Tools
Scientific Focus
- Biopharma Partners
- Patients
- Education & Events
- Login
- Contact Us

LABCORP® Plasma Focus™
Improving Outcomes for Patients with Advanced Cancers
Labcorp® Plasma Focus™ is an actionable liquid biopsy test with a clear report that focuses on FDA-approved and guideline-driven biomarkers for patients with advanced solid cancers.
For more information, please contact us using the button below.
Labcorp Plasma Focus
Please provide a few details about your practice and a Program Representative will be in touch with you shortly to help you with your inquiry.

Labcorp Plasma Focus is a guideline-driven
targeted liquid biopsy test that delivers actionable results
to guide personalized treatment when tissue is not available
Liquid Biopsy: Revolutionizing precision oncology
Watch this webinar to understand the potential clinical implications of liquid biopsies on molecular profiling of tumors for therapy selection, early detection of cancer, drug response and therapy resistance.
Focus on improving outcomes
Plasma Focus test benefits
Streamlined Experience
Simplify your tumor genomic profiling process with a convenient blood draw
Actionable Results
A highly curated gene panel that focuses on FDA-approved biomarkers
Fast Results
Personalized results within 7-10 days
Advanced Cancers
NSCLC, colorectal, breast, esophageal, gastric, gastroesophageal junction carcinomas, and melanoma
Clinical Scenarios1-2
- When obtaining tissue via biopsy is technically challenging
- When limited archived tumor tissue is available
- When rapid results are needed for treatment decisions
- When patient is progressing on current therapy
- When OmniSeq INSIGHT® tissue biopsy test result is unavailable due to specimen quality or quantity insufficiency
Test Components3
- SNVs and indels: 33 clinically actionable or relevant genes
- Amplifications: 8 genes (EGFR, ERBB2 (HER2), MET, MYC, FGFR2, KIT, CCND1, CD274)
- Translocations: 5 genes (ALK, ROS1, FGFR2, NTRK1, RET)
- MSI status: only MSI-H status will be reported
- Geocoded clinical trials: may be considered for biomarker-directed treatment
SNVs-single nucleotide variants; indels-insertions/deletions; MSI-microsatellite instability
Labcorp Plasma Focus Genes
| Gene | Translocations | Amplifications | SNV/Indels |
|---|---|---|---|
| AKT1 | no | no | yes |
| ALK | yes | no | yes |
| APC | no | no | yes |
| ARID1AC | no | no | yes |
| ATM | no | no | yes |
| BRAF | no | no | yes |
| BRCA1 | no | no | yes |
| BRCA2 | no | no | yes |
| BRIP1 | no | no | yes |
| CCND1 | no | Yes | yes |
| CD274 | no | Yes | yes |
| CD274 | no | Yes | yes |
| Gene | Translocations | Amplifications | SNV/Indels |
|---|---|---|---|
| CHH1 | no | no | yes |
| CSF1R | no | no | yes |
| EGFR | no | yes | yes |
| ERBB2 | no | yes | yes |
| EZH2 | no | no | yes |
| FGFR1 | no | no | yes |
| FGFR2 | yes | yes | yes |
| HRAS | no | no | yes |
| KIT | no | yes | yes |
| KRAS | no | no | yes |
| MET | no | no | yes |
| Gene | Translocations | Amplifications | SNV/Indels |
|---|---|---|---|
| MYC | no | yes | yes |
| NRAS | no | no | yes |
| NTRK1 | yes | no | yes |
| PDGFRA | no | no | yes |
| PIK3CA | no | no | yes |
| POLD1 | no | no | yes |
| POLE | no | no | yes |
| RAF1 | no | no | yes |
| RET | yes | no | yes |
| ROS1 | yes | no | yes |
| TP53 | no | no | yes |
Full coding region will be analyzed in all genes.
Patient report: clear and concise clinical report
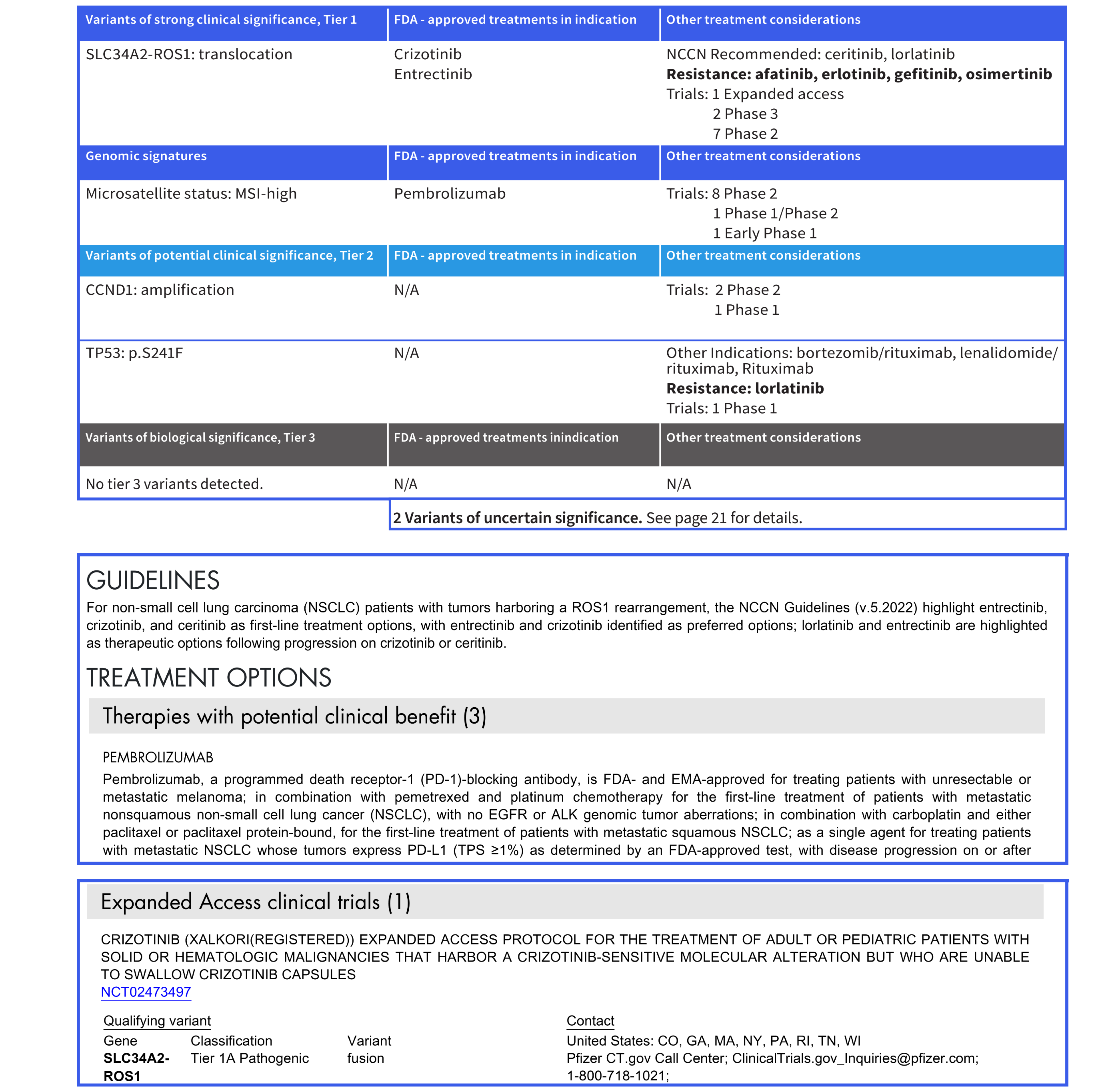
Summary of variants of strong clinical significance (Tier 1), FDA-approved therapies within and across indications and geocoded summary of clinical trials
Summary of MSI status, FDA-approved therapies and clinical trials
Summary of variants of potential clinical significance (Tier 2), other treatment considerations and summary of clinical trials
Summary of variants of biological significance (Tier 3), other treatment considerations, summary of clinical trials, and information on variants of uncertain significance
Guidelines-recommendations for patient management, summary of therapies with potential clinical benefit and therapies associated with resistance
Summary of available geocoded clinical trials
This is a sample report for informational purposes only. The final clinical report for your patient may vary in the look and layout.
Sample Requirements
- Collect a total of 20 mL of whole blood into the 2 Streck BCT® tubes supplied in the Labcorp Oncology Liquid Biopsy Kit
- Only the Labcorp Oncology Liquid Biopsy Kit can be used for collection and shipping
- Sample shipment to testing laboratory must occur within 24 hours of blood draw
Additional Documents:
Validation of the Labcorp Plasma Focus Test to Facilitate Precision Oncology Through Cell-free DNA Genomic Profiling of Solid Tumors View Article
Frequently Asked Questions
- What is the Labcorp® Plasma Focus™ test?
This is a next-generation sequencing (NGS) blood-based test for patients with advanced-stage non-small cell lung, breast, colorectal, esophageal, gastric, gastroesophageal junction cancers, and melanoma. This test evaluates 33 genes for single nucleotide variants and insertions/deletions, eight genes for amplifications, and five genes associated with translocations (fusions). Additionally, the test also assesses for microsatellite instability (MSI).
- When should a liquid biopsy be ordered?
There are several clinical scenarios to consider when ordering the Labcorp Plasma Focus test:
- Patient has a poor performance status, and obtaining a tissue biopsy is technically challenging or not safe
- Archived tumor tissue is exhausted, limited or not available
- The initial tumor biopsy was inadequate or contained insufficient tumor content
- Patient’s tumor is progressing on current therapy, and other therapeutic options are needed
- When rapid results are needed
- What are the benefits of liquid biopsy?
A liquid biopsy test facilitates comprehensive genomic profiling from a simple blood draw for patients with advanced-stage cancers. Potential benefits of a liquid biopsy test include:
- Assessment of mutations that are present in the original tumor and/or sites of metastasis
- Assessment of mutations that may develop over time and after treatment
- Ability to perform testing during disease progression to identify resistance mutations to targeted therapy
- What are the limitations of liquid biopsy?
The Labcorp Plasma Focus test should be considered a “rule-in” test instead of a “rule-out.” For example, if the test identifies a variant, we are confident that a specific alteration is present within the tumor. However, if a variant was not detected, it may be due to lack of circulating tumor DNA (ctDNA) shed into the bloodstream by the tumor. Tissue testing should be considered if a variant was not detected.
- What specific genes are sequenced by Labcorp Plasma Focus?
The Labcorp Plasma Focus test detects 33 actionable genes for single nucleotide variants and insertions and deletions. Eight genes are evaluated for amplifications (EGFR, HER2, MET, MYC, FGFR2, KIT, CCND1, CD274). Five genes are evaluated for fusions (ALK, ROS1, FGFR2, NTRK1, RET). Microsatellite instability (MSI) is also included. The complete gene list is available on our website.
- How do I interpret MSI results from a liquid biopsy assay?
The Labcorp Plasma Focus liquid biopsy can diagnose microsatellite unstable tumors (MSI-H). However, a negative result does not entirely exclude the possibility of MSI-H status (because a negative result will occur in samples that lack ctDNA).
For patients whose results are “MSI-not detected,” this could mean that circulating tumor DNA levels were below the threshold required for MSI-H detected or the tumor is microsatellite stable. To definitively diagnose microsatellite stable (MSS) tumors, tissue testing should be considered.
- How do I order Labcorp Plasma Focus?
Using the Labcorp Oncology Liquid Biopsy kit, collect 20 mL of whole blood in two Streck Cell-Free DNA tubes. Write the appropriate patient information on the tube labels and tumor requisition form, include required documentation (i.e., a recent path report, medical notes and insurance information), and ship overnight to the testing laboratory, with provided packaging, within 24 hours of the blood draw. The specimen should be maintained at room temperature. Blood samples must be received at the testing laboratory within 24 hours from the blood collection date in order to test. For questions, please contact 1-800-781-1259.
The shipping address for samples is:
PGDx*
3600 Boston Street
Suite 55
Baltimore MD 21224If you do not have a Labcorp Oncology Liquid Biopsy kit, you may request one by calling 866-875-2271 or email [email protected].
*PGDx is a subsidiary of Laboratory Corporation of America Holdings, using the brand Labcorp
- Which patients are eligible for the Labcorp Plasma Focus test?
Patients with advanced stage or recurrent, refractory non-small cell lung, breast, colorectal, esophageal, gastric, gastroesophageal junction cancers and melanoma are eligible.
- Where do I receive the results from the Labcorp Plasma Focus test?
You will receive your patients’ results through your selected Labcorp report delivery method. Should you need a copy of a report, you are welcome to contact [email protected].
For clients with a Labcorp Link™ account, results will be sent through the web-based portal. Other options include secure email or fax. Please note that a faxed version may be difficult to read.
- Is your lab CLIA Certified and CAP Accredited?
Yes. Labcorp Plasma Focus is run in a CLIA-certified and CAP-accredited lab.
- Who can I contact with additional questions?
You can contact our Client and Patient Resource team via email ([email protected]) or phone (1-800-781-1259).
Labcorp Plasma Focus Test
High Laboratory Quality Standards
CLIA and CAP accredited
References
Pennell NA, Arcila ME, Gandara DR, West H. Biomarker testing for patients with advanced non–small cell lung cancer: Real-world issues and tough choices. 2019 ASCO Educational Book. May 17, 2019.
Heitzer E, van den Broek D, Denis MG, et al. Recommendations for a practical implementation of circulating tumor DNA mutation testing in metastatic non-small-cell lung cancer. ESMO Open. 2022;7(2):100399. doi:10.1016/j.esmoop.2022.100399
Verner EL,Jackson JB, Severson E, et al. Validation of the Labcorp Plasma Focus Test to Facilitate Precision Oncology Through Cell-free DNA Genomic Profiling of Solid Tumors. J Mol Diagn. 2023 Apr 15;S1525-1578(23)00075-2. doi: 10.1016/j.jmoldx.2023.03.008.


