- Cancer Care Team
Cancer Care Team
To deliver optimal patient outcomesProducts and Services
Cancer Type
Supplies & Tools
Scientific Focus
- Biopharma Partners
- Patients
- Education & Events
- Login
- Contact Us

Introducing
Labcorp® Plasma Complete:
A liquid biopsy-based CGP solution for research and investigational use*
Access rich variant data in one low-input, non-invasive test
*Available for Investigational Use after appropriate regulatory considerations
Comprehensive genomic profiling for biomarker discovery and analysis
Intended to support research and investigational use, this robust, pan-tumor assay covering 500+ genes including SNVs, indels, select amplifications and translocations, MSI, bTMB* and LOH*, provides a comprehensive report for relevant guideline-consistent biomarkers for multiple tumor types.
*bTMB and LOH available for Research Use Only
Unlock actionable insights—even when tumor sample is limited or unavailable
Labcorp Plasma Complete analyzes circulating tumor DNA (ctDNA) to identify genetic alterations in cancer without the need for an invasive tumor biopsy.
Comprehensive panel targeting biomarkers relevant across many tumor types
521 genes
High sensitivity for high confidence variant detection
Variant detection down to 0.1%
Robust assay ensures high clinical success rate
>97% success rate
Low input ensures actionable insights from minimal sample
25 ng DNA
Flexible testing options to empower your development program
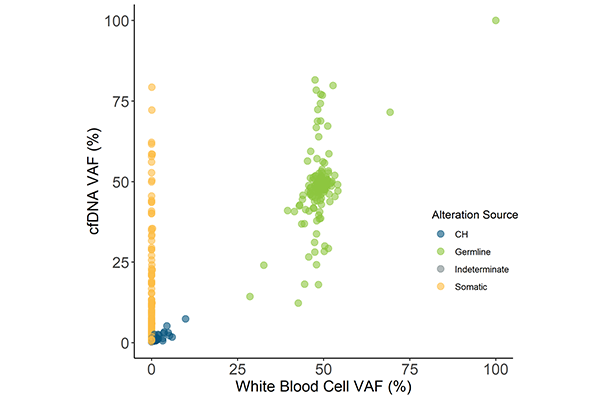
Labcorp Plasma Complete RUO with optional Matched Normal Analysis
For Research Use Only
Labcorp Plasma Complete with clinically annotated report
For Research and Investigational Use*

*Available for Investigational Use after appropriate regulatory considerations. Not for diagnostic use.
Backed by robust bioinformatics
Rapidly and accurately identify cancer mutations, empowering every study with timely and trustworthy results. High-quality training data, expert curation and machine learning algorithms combine to provide best-in-class identification of cancer mutations. Automatically curated reports highlight a wide array of high-impact, well-established and emerging biomarkers with potential clinical significance, simplifying the task of sifting through data.
A scalable platform to support biomarker-driven cancer research and clinical trials
-
Developed under ISO 13485 design control and available for research or investigational use*
-
Performed in a CAP/CLIA-certified laboratory
-
Supported by Labcorp’s global laboratory and service network
-
Distributed option available for global use
*Available for Investigational Use after appropriate regulatory considerations

Labcorp Plasma Complete assay specifications
| Specification | Labcorp Plasma Complete RUO with optional matched normal analysis | RUO Labcorp Plasma Complete with clinically annotated report | PGDx elio plasma complete RUO |
|---|---|---|---|
| Test format | Test service for Research Use Only (RUO) | Test service for Research and Investigational Use* | A distributed kit solution for global use For Research Use Only |
| Sequencing workflow | Hybrid capture | Hybrid capture | Hybrid capture |
| Analysis | Cell-free DNA (cfDNA) obtained through noninvasive blood draws | Cell-free DNA (cfDNA) obtained through noninvasive blood draws | Cell-free DNA (cfDNA) obtained through noninvasive blood draws |
| Specimen type | Plasma-derived cfDNA Buffy coat (PBMCs) optional, for matched normal analysis | Plasma-derived cfDNA | Plasma-derived cfDNA |
| Sample input and collection tube | Whole blood: 1-2 x 10 mL Streck OR K2-EDTA Plasma: Frozen (5 mL recommended; 1 mL minimum) Buffy coat: Frozen (1 mL recommended; 0.2 mL minimum) optional, for matched normal analysis | Whole blood: 2 x 10 mL Streck | Whole blood: 1-2 x 10 mL Streck OR K2-EDTA Plasma: frozen (5 mL recommended; 1 mL minimum) |
| DNA input | 25 ng recommended (10 ng minimum) | 25 ng | 25 ng recommended (10 ng minimum) |
| Alterations evaluated | Single nucleotide variants (SNVs), insertions/deletions (indels), select amplifications and translocations, MSI, bTMB†, LOH† | Single nucleotide variants (SNVs), insertions/deletions (indels), select amplifications and translocations, MSI | Single nucleotide variants (SNVs), insertions/deletions (indels), select amplifications and translocations, MSI, bTMB†, LOH† |
| Analytical sensitivity | ≥0.1% VAF | ≥0.1% VAF | ≥0.1% VAF |
| Analytical specificity | >99.99% | >99.99% | >99.99% |
*Available for Investigational Use after appropriate regulatory considerations. Not for diagnostic use.
Identify novel and emerging biomarkers with Labcorp Plasma Complete
Stay Connected


