- Cancer Care Team
Cancer Care Team
To deliver optimal patient outcomesProducts and Services
Cancer Type
Supplies & Tools
Scientific Focus
- Biopharma Partners
- Patients
- Education & Events
- Login
- Contact Us

Labcorp® Tissue Complete Service:
Powered by PGDx elio™ tissue complete
An end-to-end, sample-to-answer CGP solution for global clinical trials
Accelerate your biomarker-driven clinical trial and companion diagnostic (CDx) development with an FDA-cleared, CE-IVD-marked comprehensive genomic profiling (CGP) solution that includes complete bioinformatic analysis and dedicated customer support.
Actionable pan-tumor genomic profiling for solid tumors
Identify somatic mutations, including SNVs, indels, select amplifications and translocations, MSI and TMB
with a 505-gene panel aligned to leading professional society guideline targets.
Robust performance for limited samples
High sensitivity and specificity with minimal sample input.
Sensitive
>99%
Specific
>99%
Low input
As little as 50 ng
FFPE DNA
High success rate
Clinical success rate of >92%
A global platform to support biomarker-driven clinical trials and CDx development
Run in a global laboratory network owned and operated by Labcorp
Specialty Labs
- Baltimore, MD
- Brentwood, TN
- Buffalo, NY
- Burlington, NC
- Phoenix, AZ
- Research Triangle Park, NC
- Shelton, CT
North America
- Ann Arbor, MI
- Greenfield, IN
- Indianapolis, IN
- Los Angeles, CA
- Madison, WI
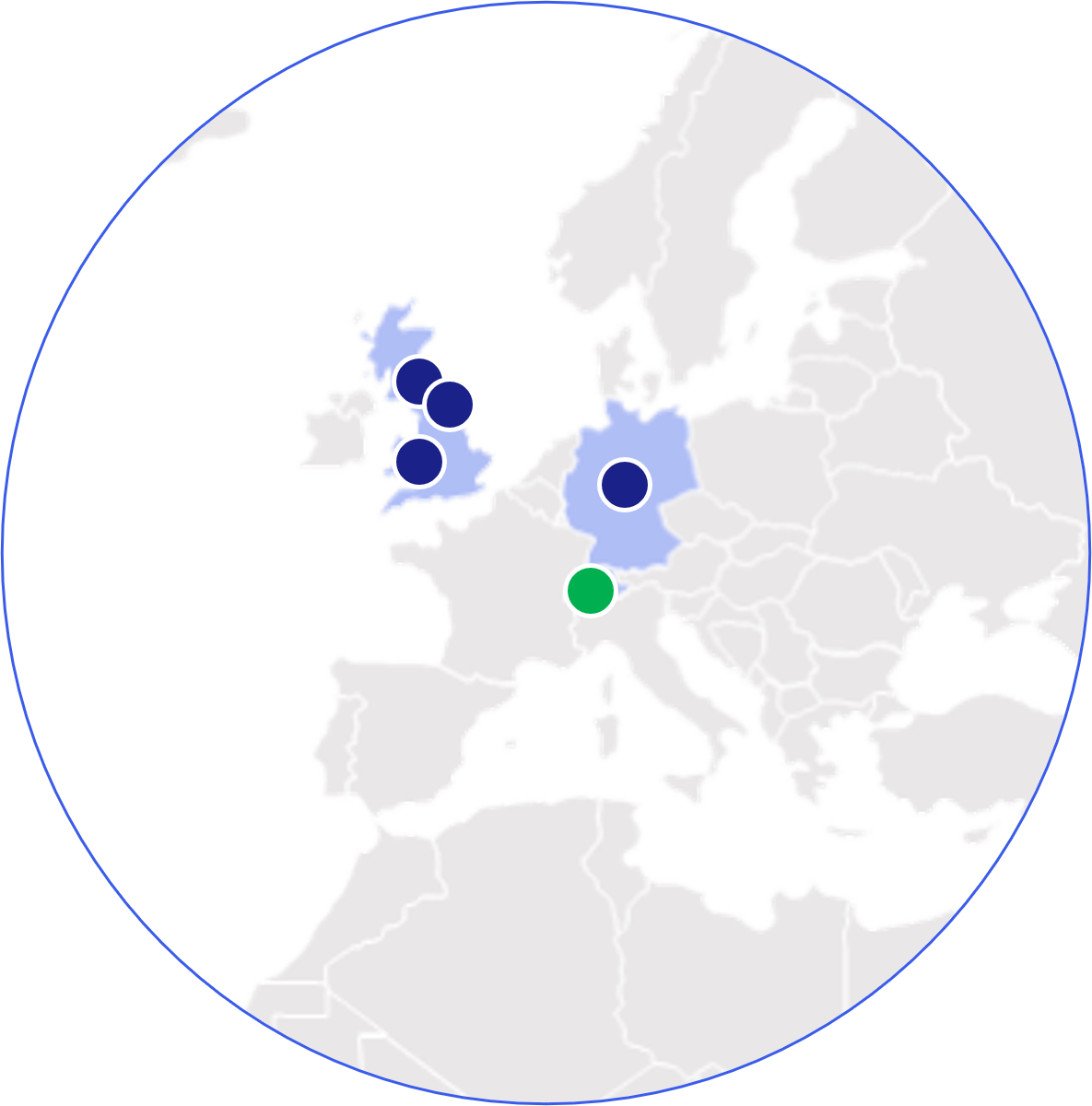
Europe
- Eye, UK
- Geneva, CH
- Harrogate, UK
- Huntingdon, UK
- Muenster, DE
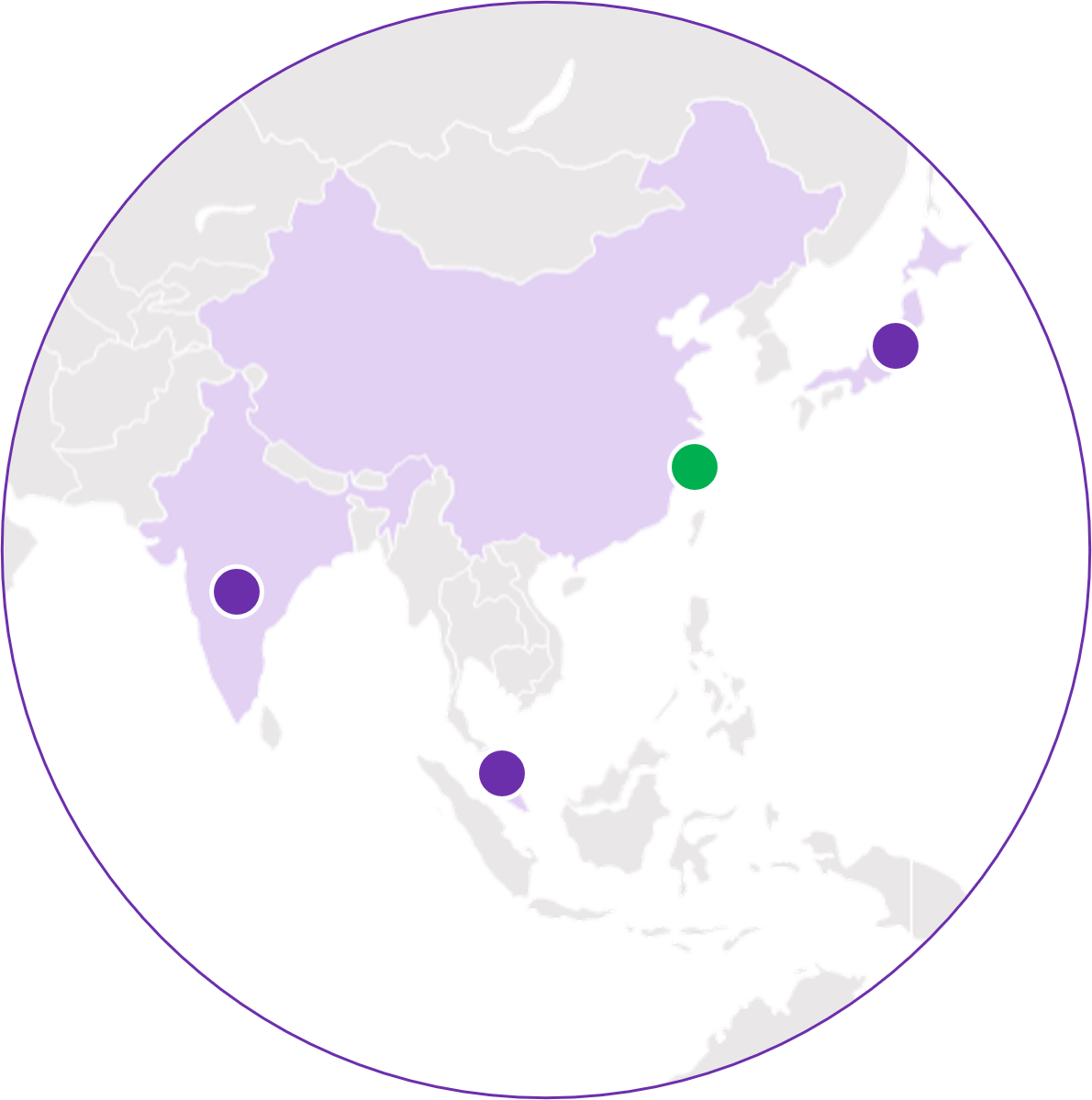
Asia/Pacific
- Bangalore, IN
- Kawagoe, JP
- Shanghai, CN
- Singapore
Labcorp Tissue Complete is available for Research Use Only in China and is not intended for diagnostic purposes.
Contact us for more information.
Rich information and detailed data reporting for research and clinical trials
-
Rapid and accurate variant detection
-
High-quality training data, expert curation and machine learning algorithms combine to provide best-in-class identification of cancer mutations
-
Automatically curated reports highlight a wide array of high-impact current and emerging biomarkers, simplifying the task of sifting through data
-
Standardized pipeline and easy-to-interpret, clearly annotated reports
-
Custom analysis and reporting available to fit your trial need

Empower your clinical trial with high-confidence variant detection
Labcorp Tissue Complete service specifications
| Specification | Labcorp Tissue Complete powered by PGDx elio™ tissue complete |
|---|---|
| Genes evaluated | 505 |
| Panel size | 2.23 Mb total (1.3 Mb TMB) |
| Sample type and input quantity | Tumor only; 100 ng DNA from FFPE (50 ng minimum) |
| Turnaround time | As few as 6-13 days |
| Average total/De-duplicated coverage | 2,300x/900x |
| Bioinformatics workflow | Single automated pipeline; custom analysis available |
| SNVs, indels, rearrangements, amplifications | Yes |
| Microsatellite Instability (MSI) | Yes |
| Tumor Mutation Burden (TMB) | Yes |
| Variant Allele Frequency (VAF) to report | 2% hotspots (5% all bases) |
| Indel detection | 40 bp insertions and deletions |
| Sample pass rate | 92.9% |
| Data analysis | Standardized |
| Data reporting | Complete Case Record, Complete Run Record, supplementary run files, custom reporting available |
A global, flexible CGP solution to empower clinical research
Stay Connected


