- Cancer Care Team
Cancer Care Team
To deliver optimal patient outcomesProducts and Services
Cancer Type
Supplies & Tools
Scientific Focus
- Biopharma Partners
- Patients
- Education & Events
- Login
- Contact Us
HT-29 as a preclinical model for colorectal cancer
October 1, 2016
Author: Maryland Franklin, PhD, Vice President, Scientific Development
Date: October 2016
For men and women combined, colorectal cancer (CRC) is the second leading cause of cancer-related deaths in the United States. The American Cancer Society estimates that over 49,000 individuals will die of CRC in 2016. Fortunately, the death rate from CRC has been declining in both men and women over the past several decades. Early screening efforts along with improved treatment options are at least two of several likely reasons for this drop. However, while there are now more than one million CRC survivors in the United States, we will see over 95,000 new cases being diagnosed in 2016.
Treatment options for CRC are highly dependent on the stage of disease, but many patients receive cocktails of chemotherapies and in some cases radiation treatment along with chemotherapy. A number of preclinical xenograft models exist that allow pharmaceutical and biotech companies to investigate new treatment approaches for CRC. We have a number of CRC models (see Table 1) and HT-29 represents one of our most highly utilized lines. Figure 1 illustrates growth of the line following subcutaneous implant in nude mice. Most studies will stage within two weeks following implant and the tumor doubles roughly every six to seven days. We have used this model to test a number of chemotherapies, as shown in Figures 2-4.
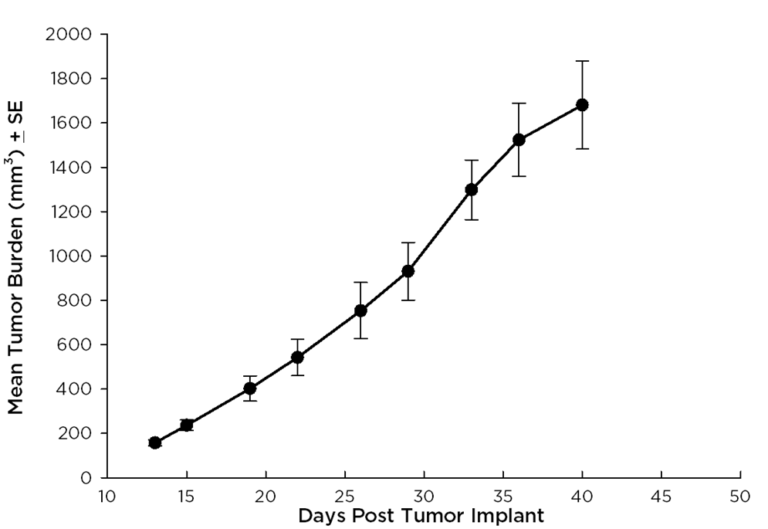
Fig. 1: Subcutaneous Growth of HT-29 Fragments Implanted Into nu/nu Mice
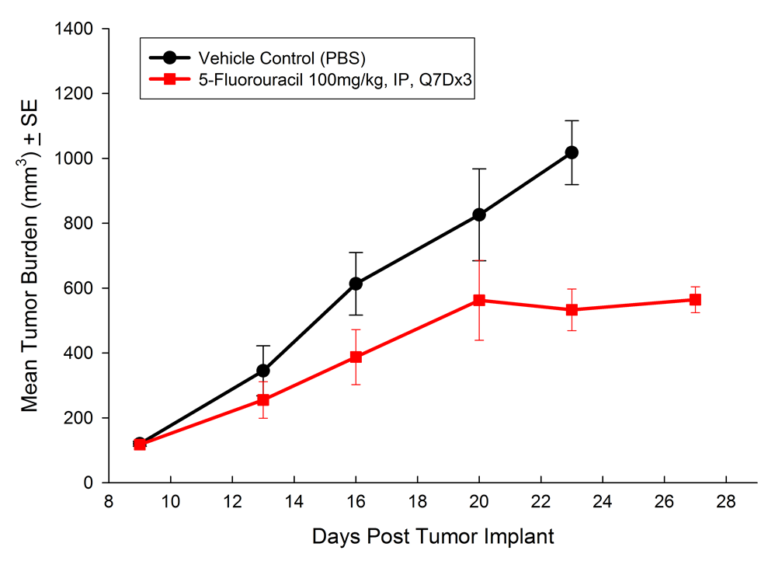
Fig. 2: Subcutaneous Growth of HT-29 Following Treatment with 5-Fluorouracil
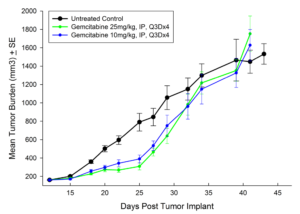
Fig. 3: Subcutaneous Growth of HT-29 Following Treatment with Gemcitabine
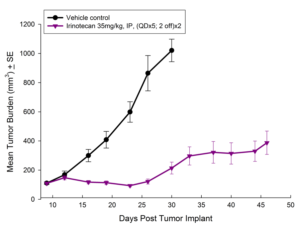
Fig. 4: Subcutaneous Growth of HT-29 Following Treatment with Irinotecan
In this model we have also evaluated the combination of radiation and gemcitabine (Figure 5) and illustrate the added benefit of the combination over either single agent treatment.
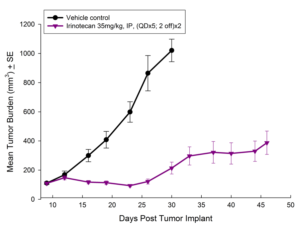
Fig. 5: Subcutaneous Growth of HT-29 Following Combination Treatment with Localized Radiation and Gemcitabine
Contact us to discuss your next preclinical CRC study design.


