- Cancer Care Team
Cancer Care Team
To deliver optimal patient outcomesProducts and Services
Cancer Type
Supplies & Tools
Scientific Focus
- Biopharma Partners
- Patients
- Education & Events
- Login
- Contact Us
Pan02 – pancreatic ductal adenocarcinoma model characterized for immuno-oncology applications
July 1, 2017
Author: Dylan Daniel, PhD, Director, Scientific Development
Date: July 2017
Pancreatic ductal adenocarcinoma (PDAC) is the most prevalent form of pancreatic cancer representing about 95% of all cases. In 2017, approximately 50,987 people will be diagnosed with PDAC in the United States, and approximately 40,936 patient deaths will occur, making PDAC one of the most lethal forms of cancer. Combination anti-metabolite and anti-mitotic taxane based chemotherapy is the standard of care in the US, but these treatment options historically only improve overall survival by weeks. There is a critical unmet medical need for novel therapeutic approaches to treat pancreatic cancer.
Because so many novel approaches to treat pancreatic cancer have met with disappointing clinical outcomes, many drug discovery scientists have turned to immunotherapy in pancreatic cancer based on the recent successes of harnessing the immune system to treat other cancers. Although there are a limited number of syngeneic mouse pancreatic cancer cell lines, Labcorp has characterized the Pan02 PDAC model for immuno-oncology applications. Pan02 was derived from C57BL/6 mice given orthotopic 3-methyl-cholanthrene and is refractory to many standard chemotherapeutic agents.1 Pan02 contains a loss of function mutation in the SMAD4 gene that is functionally similar to inactivating mutations in approximately 30% of human pancreatic cancers.2
Mean and Individual Growth Curves
Labcorp maintains Pan02 as a transplantable fragment model, and its mean growth kinetics (Figure 1A) and individual animal growth curves (Figure 1B) are illustrated. Pan02 has a mean tumor doubling time of six days which is slower than most syngeneic mouse cell line tumors. This slower growth may make it more tractable for immunotherapy since there is time for therapeutics to modify the immune system and elicit anti-tumor activity prior to tumors reaching euthanasia criteria.
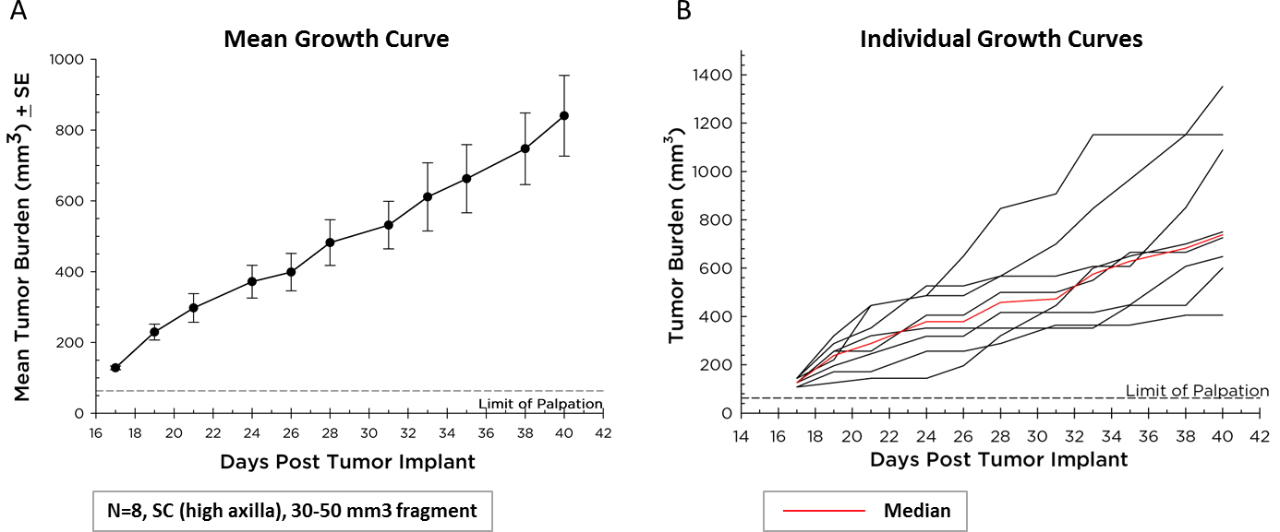
Fig. 1 (A&B) – Pan02 Pancreatic Cancer Growth in C57BL/6 Mice
Anti-PD-1 and Anti-CTLA-4 Efficacy and Survival Rates
Currently, T cell checkpoint inhibitor antibodies against CTLA-4, PD-1, and PD-L1 are approved in non-pancreatic cancer settings. We tested anti-CTLA-4 (clone 4F10) and anti-PD-1 (clone RMP1-14), singly and in combination, in the Pan02 model. Dosing was initiated three days after implant and mean group tumor burden illustrates no significant activity in any groups (Figure 2A). It is often the case that immunotherapies may reveal activity in only a subset of animals under treatment so individual animal plots are valuable to detect those responders. However, the individual animal plots do not reveal any significant activity by the test agents that isn’t also observed in the control group (Figure 2B). Further, as expected based on growth response data, there is no improvement in survival in the treatment arms relative to control (Figure 3).
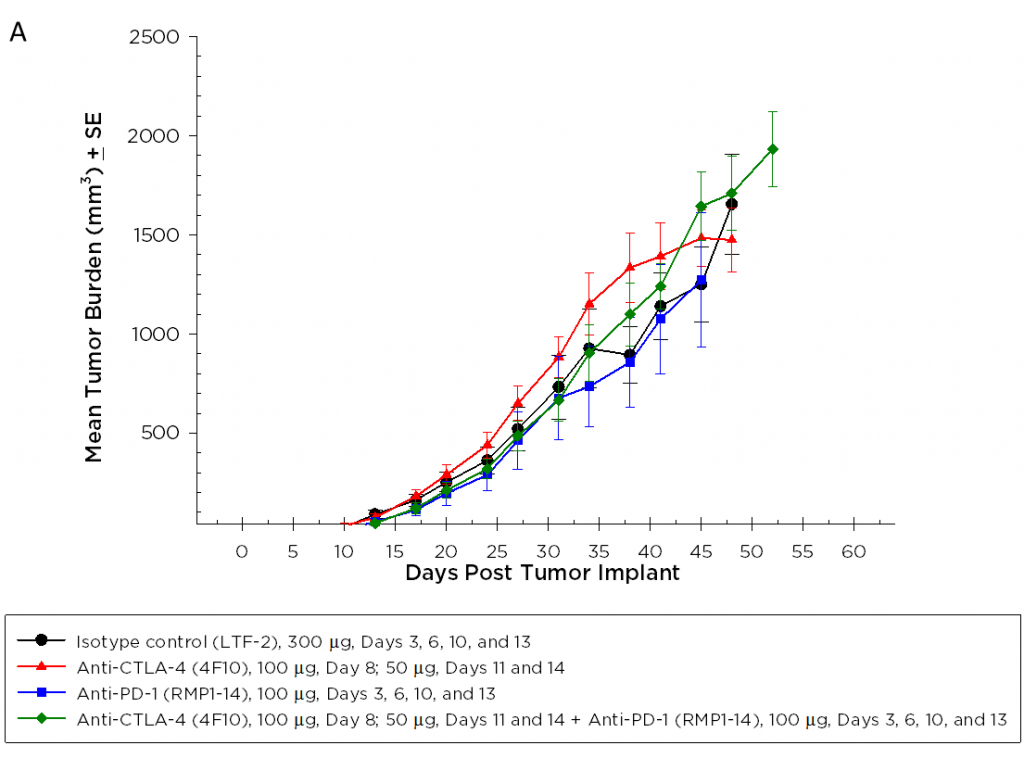
Fig. 2A – Efficacy of Anti-PD-1 and Anti-CTLA-4 Against Pan02 Pancreatic Tumors
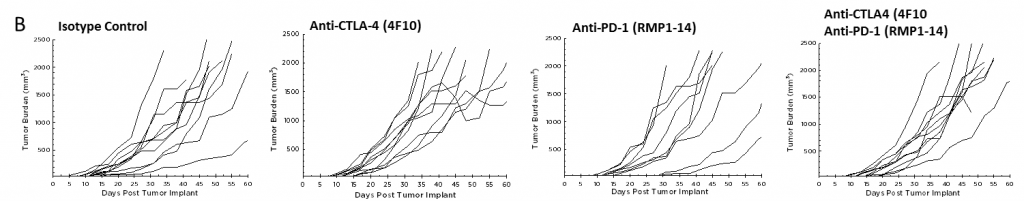
Fig. 2B – Efficacy of Anti-PD-1 and Anti-CTLA-4 Against Pan02 Pancreatic Tumors
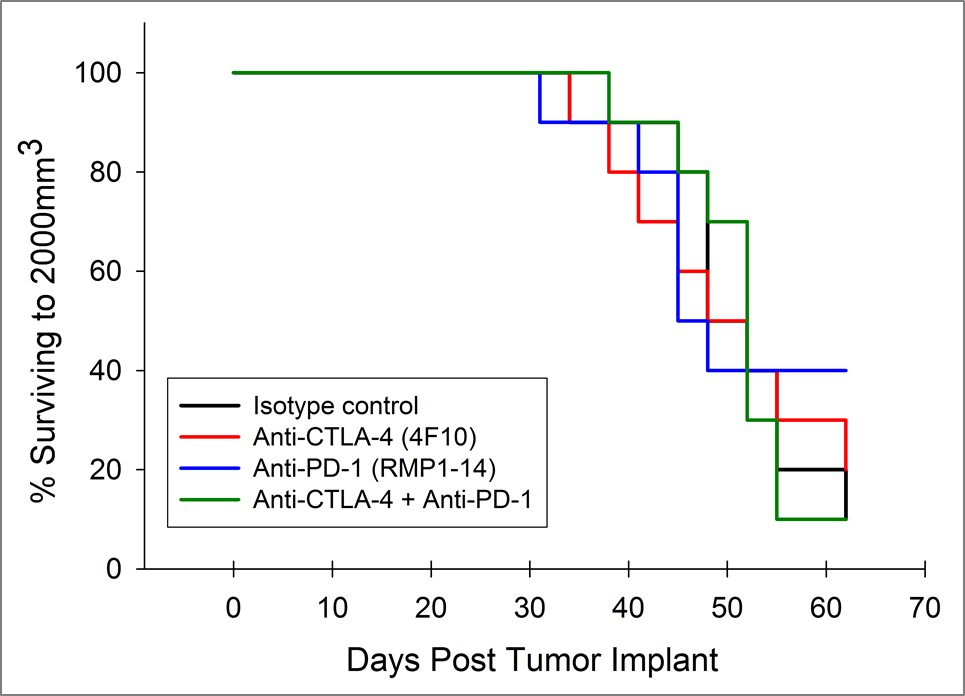
Fig. 3: Survival of Pan02 Pancreatic Tumor Bearing Mice Treated with Anti-PD-1 and Anti-CTLA-4
Immune Profiling
We wanted to determine if the checkpoint inhibitors had any impact on the immune cell infiltration in the Pan02 tumors. In Figure 4, the immune profiles for total CD45+ leukocytes, CD4+ T cells, CD8+ T cells, regulatory T cells (Tregs), monocytic myeloid derived suppressor cells (M-MDSC), and granulocytic MDSCs (G-MDSC) are shown.
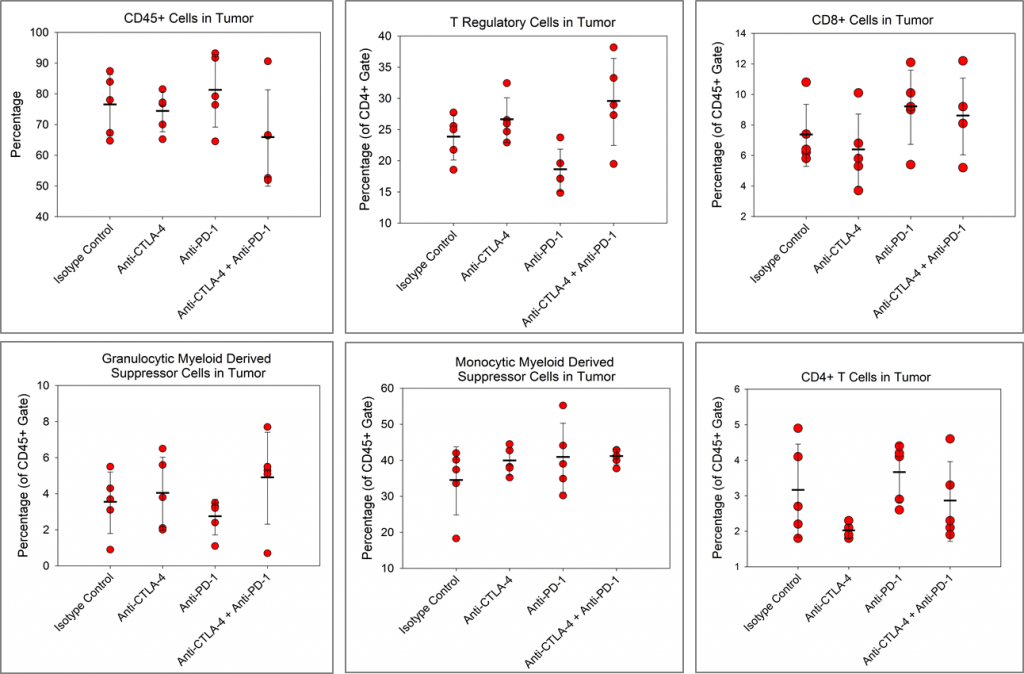
Although, there is a trend toward reduced CD4+ T cells in the anti-CTLA-4-treated group and a trend toward reduced Tregs in the anti-PD-1 treated group relative to isotype control, no other immune changes are observed. Despite checkpoint inhibitors alone having no apparent activity in the Pan02 model, there are published reports of checkpoint inhibitor activity in Pan02 tumors with radiation3 or a costimulatory agonist anti-CD40 antibody.4 Importantly, anti-PD-L1 strongly synergized with both anti-CD40 and radiation in those studies indicating that while Pan02 tumors are refractory to single agent checkpoint inhibitors, checkpoint inhibition can promote activity of combination partners.
Contact us to speak with one of our scientists to see how Pan02 or one of our other syngeneic models can be used for your next immuno-oncology study.


