- Cancer Care Team
Cancer Care Team
To deliver optimal patient outcomesProducts and Services
Cancer Type
Supplies & Tools
Scientific Focus
- Biopharma Partners
- Patients
- Education & Events
- Login
- Contact Us
LL/2: an immunosuppressive murine tumor model
October 1, 2019
Author: Maryland Franklin, PhD | Vice President, Scientific Development
Date: October 2019
Lung cancer is the second most common cancer diagnosed in both men and women in the United States and is, by far, the most common cause of cancer-related deaths in men and women. In 2019, the American Cancer Society estimates that 228,150 new cases of lung cancer (116,440 in men and 111,710 in women) will be diagnosed, and 142,670 deaths from lung cancer (76,650 in men and 66,020 in women) will occur.
The number of deaths caused by lung cancer peaked in 2005 at 159,292 and has been gradually declining ever since.[1] However, the five-year lung cancer survival rate is only around 20% and is considerably lower than other cancers. If lung cancer is detected early, the five-year survival climbs to ~56%, but only ~16% of lung cancers are detected that early. For later stage detection the five-year survival rate can be as low as 5%.
There are many treatment options for lung cancer, depending on which type of lung cancer one is diagnosed with and what stage of disease is present. Everything from surgery, chemotherapy, radiation, targeted therapy, and immunotherapy are being used clinically. While there are some successes, especially in patients with certain gene mutations and more recently with newer immunotherapies, the overall prognosis is still poor. Thus, the continual development of new treatments for lung cancer is needed.
The advent of immunotherapy has necessitated syngeneic mouse tumor models to further advance the development of immuno-oncology treatments. One of these models is the LL/2 (Lewis Lung) lung carcinoma model that has been characterized by Labcorp to support development of novel agents. The LL/2 cell line was developed from a primary tumor nodule from the Lewis lung carcinoma model that spontaneously developed as an epidermoid carcinoma in the lung of a C57BL mouse. As shown below, LL/2 is classified as a “cold” tumor with low infiltration of T cells and high infiltration of myeloid suppressive cells. As many cancer patients are non-responsive to current immunotherapies, understanding “cold” tumor models is an important part of developing and utilizing syngeneic mouse approaches for drug discovery and development.
LL/2 Tumor Immune Profile
Baseline immune profile of LL/2 tumor infiltrates was determined on 6 untreated tumors (~500mm3) analyzed with the Labcorp CompLeukocyteTM Package. Of the CD45+ cells infiltrating these tumors, the M-MDSCs represented the largest cell population (37%) followed by G-MDSCs (22%) and M2 TAMs (20%). T cells (both CD8+ and CD4+), along with M1 TAMs, B cells, NK, NKT and dendritic cells were minimally represented (Fig. 1). The overall profile is suggestive of a non-immunogenic model.
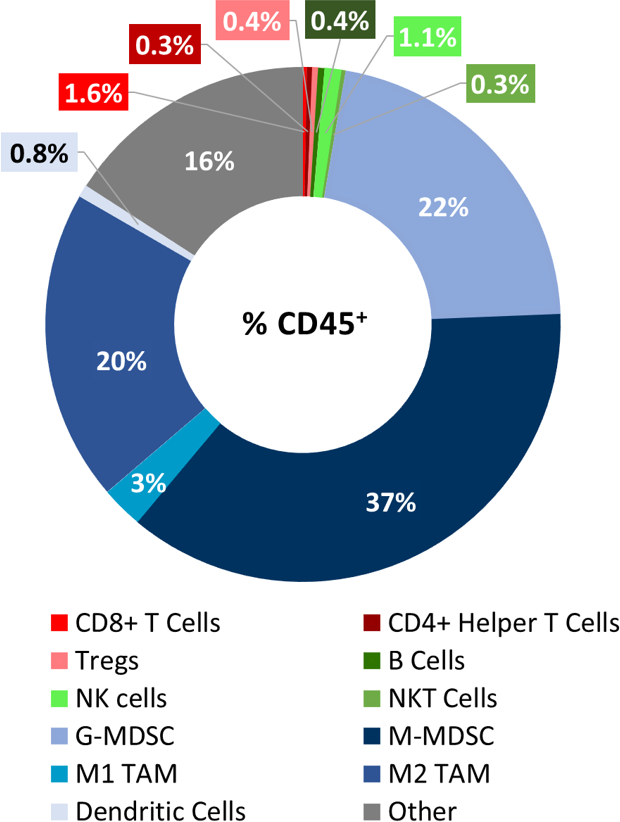
Fig. 1: Immunophenotyping of tumor immune cell infiltrates in the LL/2 model.
LL/2 Growth and Response to Therapy
The in vivo doubling time of subcutaneous (SC) LL/2 tumors is very rapid at approximately 2.5-3 days. This results in a model which can facilitate up to a two-week dosing window for test agents to elicit their anti-tumor activity. The model itself does not result in reduction of body weight (Fig. 2A, 2B).
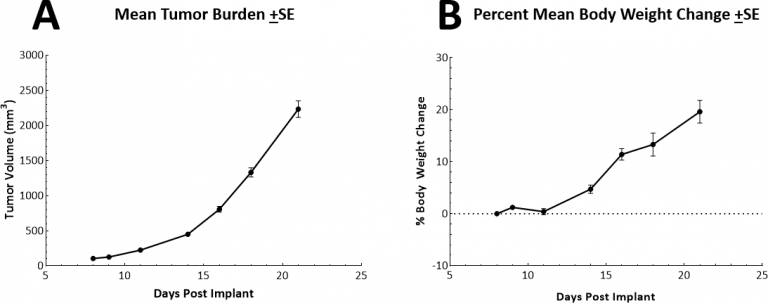
Fig. 2: Growth and body weight change following SC implant of LL/2 cells.
There are a variety of chemotherapy approaches for lung cancer and to this end we have tested both doxorubicin and paclitaxel as single agents in the LL/2 model. Doxorubicin is an anthracycline that exhibits broad spectrum anti-tumor activity and paclitaxel is a tubulin-binding agent that is widely used for the treatment of non-small cell lung cancer (NSCLC). In the LL/2 model, neither test agent demonstrated meaningful anti-tumor responses at the dose and schedules we examined (Figs. 3A & B).
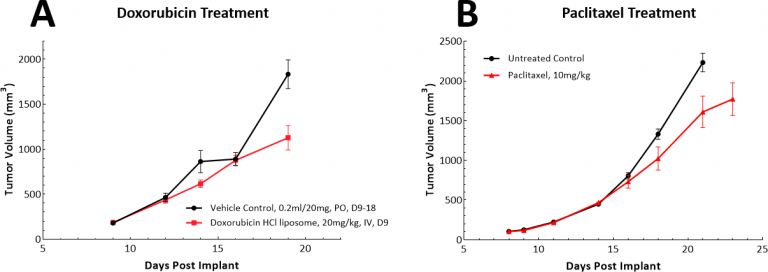
Fig. 3: Response of LL/2 SC tumors to single agent doxorubicin or paclitaxel treatment.
With the advent of immunotherapy, we have also examined whether the LL/2 model would respond to single agent checkpoint inhibitors, anti-PD-1 or anti-PD-L1 (Fig. 4). Fig. 4 demonstrates mean tumor volumes (Fig. 4A) and individual tumor volumes (Figs. 4B, C, & D) following treatment with isotype control, anti-PD-1 or anti-PD-L1. Dosing with all test agents began once tumors were established (~100mm3). Not surprisingly, neither test agent showed therapeutic benefit due to the highly immunosuppressive tumor microenvironment in this model. We then went on to postulate whether the combination of paclitaxel with immune checkpoint inhibitors would provide combination benefit (Figs. 5A & B). However, this approach did not lead to improved outcome for the mice fully solidifying the position that the model has a “cold” phenotype. It is possible that triple combination approaches could provide benefit in this model and those looking for approaches to manage “cold” tumors would find this model useful.
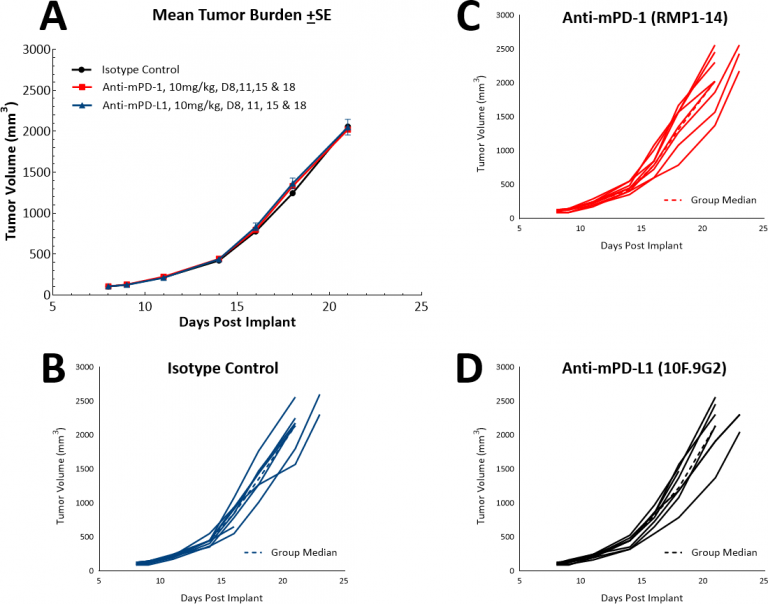
Fig. 4: Response of LL/2 SC tumors to single agent checkpoint inhibitors anti-mPD-1 and anti-mPD-L1.
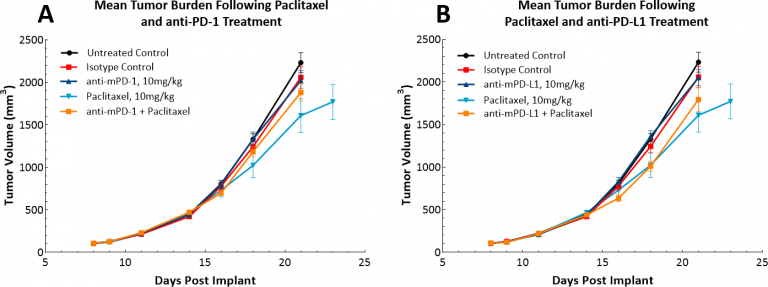
Fig. 5: Response of LL/2 SC tumors to combination treatment with checkpoint inhibitors and paclitaxel.
LL/2 Tumors Following Localized Radiation Therapy Alone or in Combination with Anti-PD-1
In lung cancer patients, radiation therapy is commonly utilized both alone, as a palliative monotherapy, and in combination with chemotherapy. While focal radiation is highly recommended, we have utilized our RadSource RS-2000 to deliver localized radiation in a few models. In the subcutaneous LL/2 model we tested a single high dose (20Gy) of localized radiation to mice with SC tumors implanted in the sacral region. We found that radiation alone increased the time to evaluation size to 36 days from 20 days in the control group. With the addition of anti-PD-1 the time to evaluation increased marginally to 38.5 days, suggesting no added benefit of the combination treatment (Fig. 6). However, a triple combination of radiation, immune checkpoint blockade, and a novel therapy could be further tested in this model.
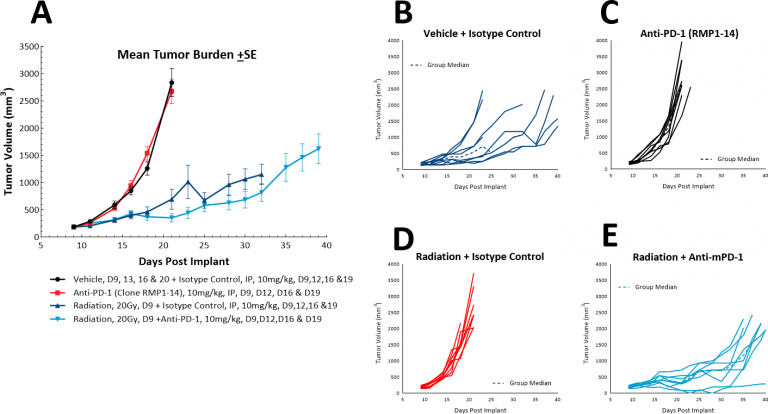
Fig. 6: Response of LL/2 SC tumors to combination treatment with localized radiation and anti-PD-1.
Subcutaneous tumor models are useful for quick and relatively easy assessments of anti-tumor activity. However, by the nature of the implant location, these tumors may not replicate all the needed biology that would more closely represent the disease in the patient. To this end, we have a luciferase-enabled version of LL/2, LL/2-Luc-M38, that is being used to develop the LL/2 model in the orthotopic lung setting.
The LL/2 murine lung carcinoma model can be employed as a preclinical immuno-oncology model. Our data supports the use of this tool in investigating novel treatment combinations with radiation or checkpoint inhibitors, or other novel approaches to the treatment of “cold” non-immunogenic tumors.
Please contact us to speak with our scientists about how LL/2 or one of our other syngeneic models can be used for your next immuno-oncology study.


