- Cancer Care Team
Cancer Care Team
To deliver optimal patient outcomesProducts and Services
Cancer Type
Supplies & Tools
Scientific Focus
- Biopharma Partners
- Patients
- Education & Events
- Login
- Contact Us

Improving Outcomes for Patients with Myeloid Malignancies
IntelliGEN® Myeloid is a next generation sequencing (NGS) assay that evaluates 50 genes known to be associated with myeloid malignancies

To better serve the evolving needs of hematologic malignancy testing, we are introducing three new NGS panels: Labcorp Myeloid NGS, Labcorp Lymphoid NGS and Labcorp Pan-Heme NGS. As part of this update, IntelliGEN® Myeloid will be discontinued on May 19, 2025.
We are committed to making this transition as smooth as possible and appreciate you continuing to choose Labcorp. If you have questions regarding pricing or other details, please contact your sales representative or client services team at the following numbers based on your laboratory of choice:
For Phoenix and Brentwood laboratories: 800-710-1800
For Shelton laboratory: 800-447-5816
For the CMBP laboratory: 800-366-7230
Powering Better Decisions
The IntelliGEN Myeloid panel identifies somatic mutations useful in providing diagnostic, prognostic, and predictive information for patients with myeloid malignancies including Myelodysplastic Syndromes (MDS), Acute Myeloid Leukemia (AML), and Myeloproliferative Neoplasms (MPN)
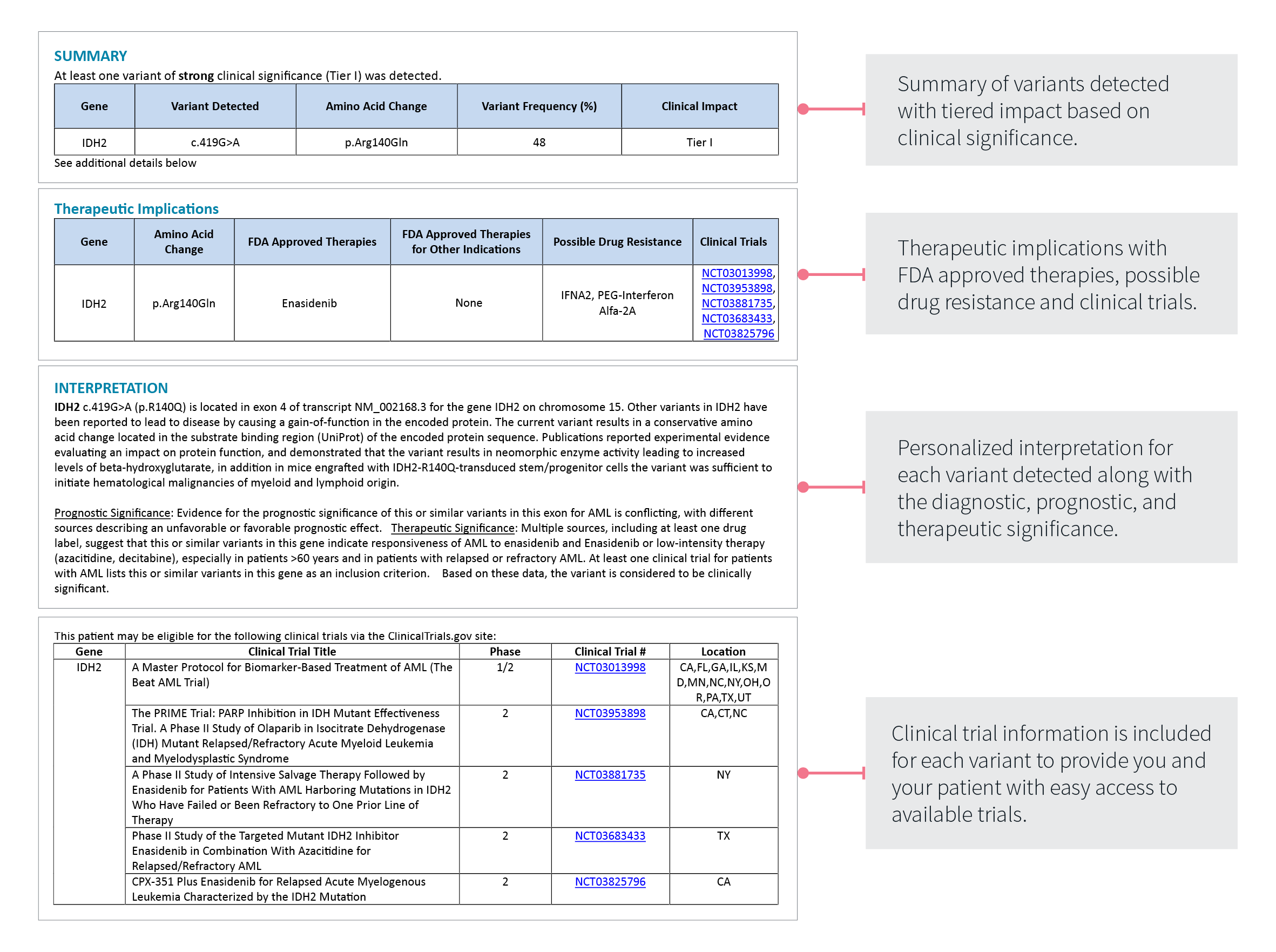
Patient Reports: Concise, Comprehensive & Actionable
Comprehensive Quick-Read Results
Gain actionable insights at a glance with:
- Clinically significant results
- Therapy implications summary
- Personalized interpretation for each variant
THERAPEUTIC IMPLICATIONS
Start to formulate a plan with:
- FDA-approved therapies
- Possible drug resistance
- Clinical trial information for each variant
PERSONALIZED INTERPRETATION:
Expand your patient’s options with:
- Diagnostic, prognostic, and therapeutic significance
- Clinical trial matches for each variant
- Easy access to available trial information
IntelliGEN Myeloid Panel Genes
| Gene* | Association | ||
|---|---|---|---|
| MDS | AML | MPN | |
| ABL1 | diagnostic and/or prognostic significance in AML | diagnostic and/or prognostic significance in MPN | |
| ASXL1 | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | diagnostic and/or prognostic significance in MPN |
| BCOR | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | diagnostic and/or prognostic significance in MPN |
| BCORL1 | diagnostic and/or prognostic significance in AML | ||
| BRAF | diagnostic and/or prognostic significance in MPN | ||
| CALR | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in MPN | |
| CBL | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in MPN | |
| CDKN2A | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | |
| CEBPA | diagnostic and/or prognostic significance in AML | ||
| CSF3R | diagnostic and/or prognostic significance in AML | diagnostic and/or prognostic significance in MPN | |
| CUX1 | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | |
| DNMT3A | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | diagnostic and/or prognostic significance in MPN |
| ETV6 | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | diagnostic and/or prognostic significance in MPN |
| EZH2 | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | diagnostic and/or prognostic significance in MPN |
| Gene* | Association | ||
|---|---|---|---|
| MDS | AML | MPN | |
| FBXW7 | |||
| FLT32,3 | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | |
| GATA1 | diagnostic and/or prognostic significance in AML | ||
| GATA2 | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | |
| IDH14 | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | diagnostic and/or prognostic significance in MPN |
| IDH25 | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | diagnostic and/or prognostic significance in MPN |
| IKZF1 | diagnostic and/or prognostic significance in AML | diagnostic and/or prognostic significance in MPN | |
| JAK2 | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in MPN | |
| JAK3 | diagnostic and/or prognostic significance in AML | ||
| KDM6A | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | diagnostic and/or prognostic significance in MPN |
| KIT1 | diagnostic and/or prognostic significance in AML | ||
| KMT2A | diagnostic and/or prognostic significance in AML | ||
| KRAS | diagnostic and/or prognostic significance in AML | ||
| MPL | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in MPN | |
| Gene* | Association | ||
|---|---|---|---|
| MDS | AML | MPN | |
| NF1 | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | |
| NOTCH1 | |||
| NPM1 | diagnostic and/or prognostic significance in AML | ||
| NRAS | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | |
| PDGFRA1 | diagnostic and/or prognostic significance in AML | ||
| PHF6 | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | |
| PML | diagnostic and/or prognostic significance in AML | ||
| PTEN | diagnostic and/or prognostic significance in AML | ||
| PTPN11 | diagnostic and/or prognostic significance in AML | ||
| RAD21 | diagnostic and/or prognostic significance in AML | ||
| RUNX1 | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | diagnostic and/or prognostic significance in MPN |
| SETBP1 | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | diagnostic and/or prognostic significance in MPN |
| SF3B1 | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | diagnostic and/or prognostic significance in MPN |
| SMC1A | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | |
| Gene* | Association | ||
|---|---|---|---|
| MDS | AML | MPN | |
| SMC3 | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | |
| SRSF2 | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in MPN | |
| STAG2 | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | |
| TET2 | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | diagnostic and/or prognostic significance in MPN |
| TP53 | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | |
| U2AF1 | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | |
| WT1 | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in AML | |
| ZRSR2 | diagnostic and/or prognostic significance in MDS | diagnostic and/or prognostic significance in MPN | |
| Genes associated with FDA approved therapies |
*Identification of mutations should always be used within the context of clinical findings and bone marrow evaluation
- GLEEVEC® (imatinib mesylate). Novartis Pharmaceuticals Corporation East Hanover, NJ; 2019.
- RYDAPT® (midostaurin). Novartis Pharmaceuticals Corporation, East Hanover, NJ; 2021.
- XOSPATA® (gilteritinib). Astellas Pharma US, Inc., Northbrook, IL; 2021
- TIBSOVO® (ivosidenib). Servier Pharmaceuticals LLC., Boston, MA; 2021.
- IDHIFA® (enasidenib). Celgene Corporation, a Bristol Myers Squibb company, Summit, NJ; 2021.
| Sample requirements |
|---|
Please provide a clinical indication or related ICD-10 code on the test requisition form Specimen type: Whole blood, bone marrow, cell pellets from whole blood or cell pellets from bone marrow Volume: 3-5ml (whole blood); 1-2ml (bone marrow) Container: Lavender-top (EDTA) tube or green-top (heparin) tube |
| Turnaround time: 10-14 Days |
For additional test information, please visit the test menu page
High Laboratory Quality Standards
- NYS CLEP approved
- CLIA and CAP accredited
Labcorp Broad National Coverage
- In-network with most major health plans
- 90% of patients paid $0*
*Based on managed care claim data/internal Labcorp billing data of over 1,729 patients in 2021