- Cancer Care Team
Cancer Care Team
To deliver optimal patient outcomesProducts and Services
Cancer Type
Supplies & Tools
Scientific Focus
- Biopharma Partners
- Patients
- Education & Events
- Login
- Contact Us
BT-474 Human ductal breast carcinoma: New life for an old model
May 1, 2020
Author: Erin Trachet
Date: May 2020
Introduction
The BT-474 cell line has a long history, but many of us thought it had run its natural course and was quickly becoming obsolete.
The American Type Culture Collection (ATCC) reports that the BT-474 cell line was originally established in 1978 by E. Y. Lasfargues, who obtained a tumor biopsy from a 60-year-old woman with advanced stage invasive ductal breast carcinoma. The cell line was later classified as estrogen receptor positive (ER), progesterone receptor positive (PR) and human epidermal growth factor receptor 2 positive (HER2) (1). This information was critical in linking the BT-474 model used in preclinical breast cancer research to real patients with the same genetic profile.
According to the American Association of Cancer Research, about 80% of all breast cancer patients are ER positive and ~65% are PR positive. Patients with breast cancer that is both ER and PR positive are more likely to respond to hormone therapy. Furthermore, ~20% of all breast cancers show an increase in HER2 expression, which is linked to aggressive and fast-growing tumors. The connection between the BT-474 preclinical model and patient biomarkers, coupled with the dire need for continual advancements in breast cancer treatment, made this model highly desirable for both in vitro and in vivo research.
The BT-474 Challenge
This all seemed too good to be true, and it was. The issue with BT-474 (and several other breast cancer models) is that it is notoriously problematic to work with in vivo.
When implanted subcutaneously, or into the mammary fat pad of female nude mice, the tumor growth rate is extremely slow (tumor doubling time of >20 days) with significant tumor growth variability and spontaneous tumor regressions. These results are consistent across several studies despite attempts to adjust multiple variables such as mouse strain (SCID or nude), implant material (tumor fragments or cells), cell implant supplements (Matrigel® or brain feeder layer) and hormone supplement (estradiol).
These issues make BT-474 a very challenging model to work with, and scientists and researchers often have to find other models with similar genetic profiles that are easier to work with and can produce more reliable data.
However since there are very few models that meet all the criteria that BT-474 does (human breast carcinoma that is ER+, PR+ and HER2+), we decided to do some additional work to continue optimizing the model. Additionally, the advancement and access to NSG mice allows us to evaluate growth of BT-474 in this most immune deficient strain.
Analysis
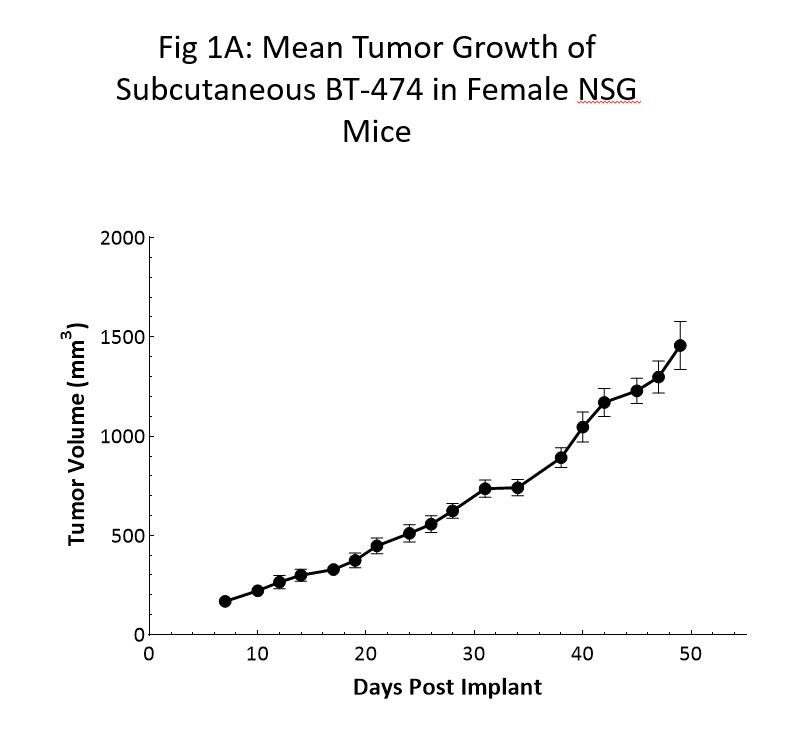
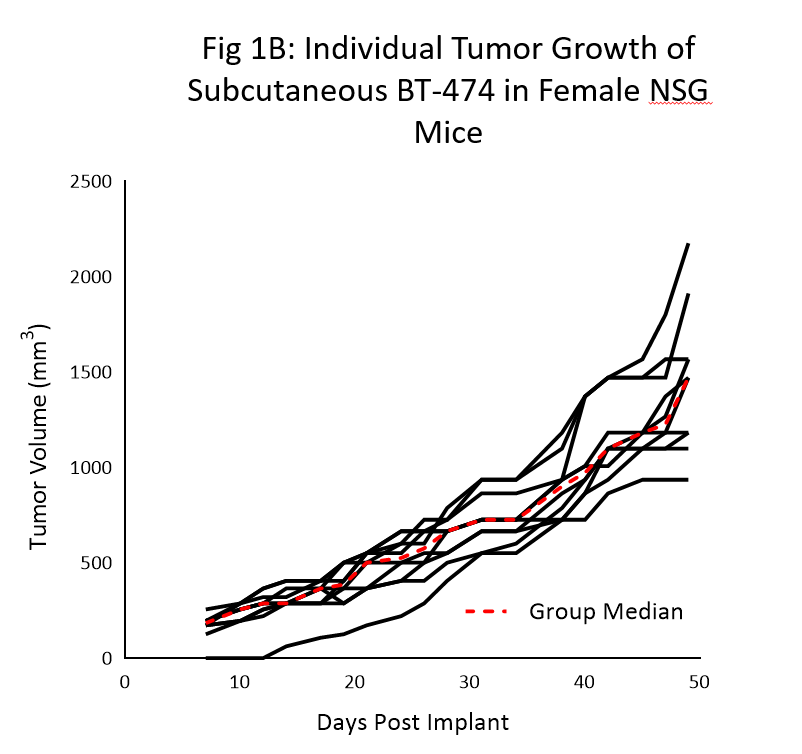
Tumor growth kinetics for the BT-474 model in NSG mice is shown in Figure 1. The median doubling time is ~12 days with a steady increase in tumor volume with no apparent tumor related body weight loss (data not shown). The time to 150mm3 (established tumors) was 7 days post implant and the time to 1000mm3 (evaluation size) was 40 days post implant.
Similar growth kinetic data has been reproduced in a handful of studies over the last 6 months.
As the data clearly shows, the utilization of the NSG mouse was crucial in the newfound reliable and reproducible growth of this model.
Response to HER2 Targeted Treatment
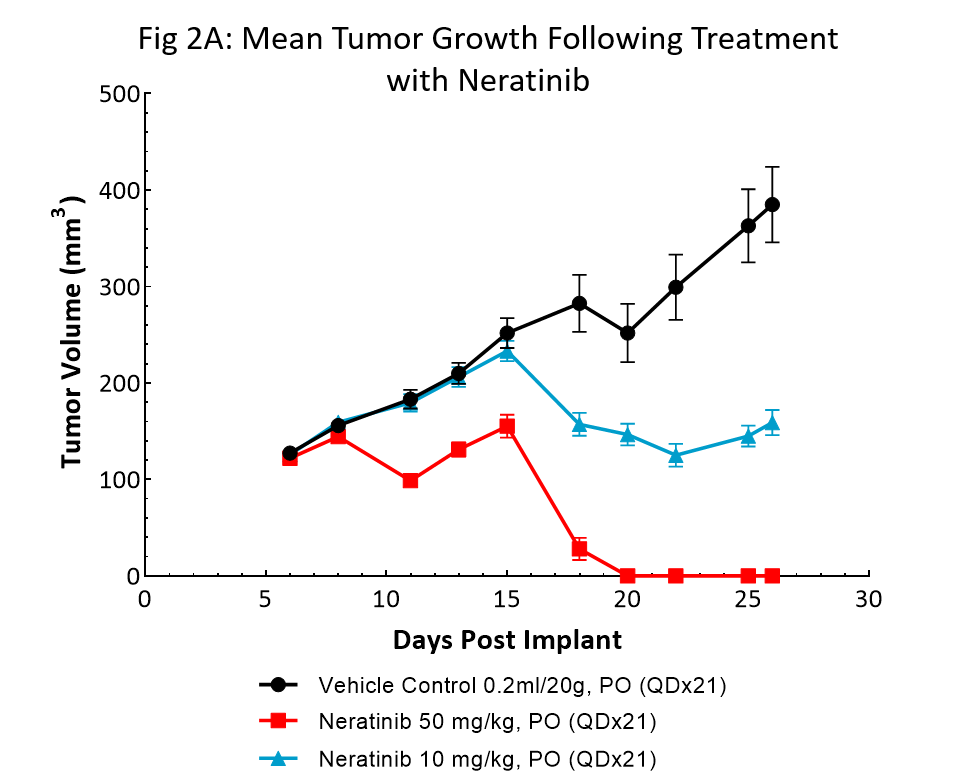
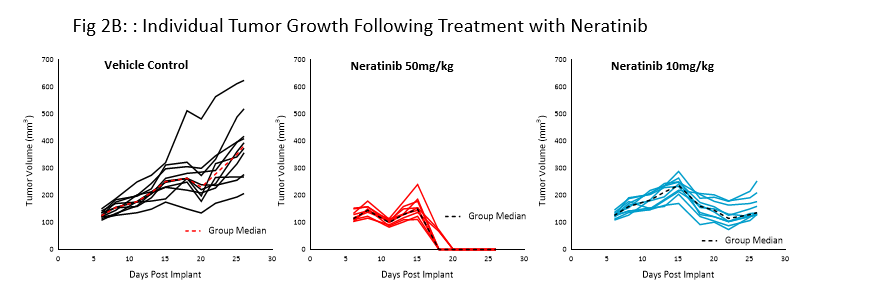
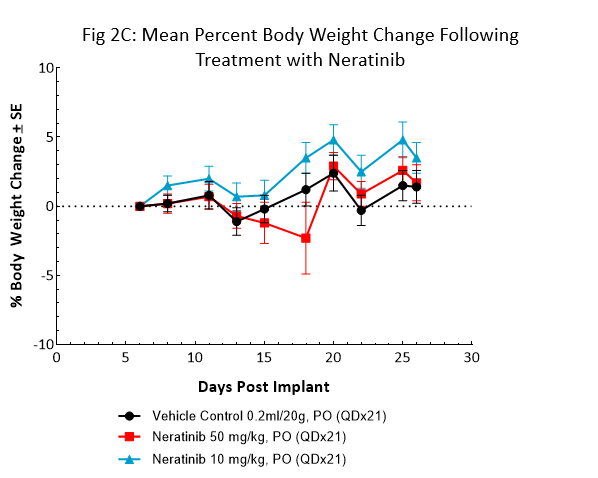
Neratinib (Nerlynx®) is an FDA approved, orally available, irreversible inhibitor of the receptor tyrosine kinases Her2 (human epidermal growth receptor 2) and EGFR (epidermal growth factor receptor). Response to neratinib at both 10 and 50mg/kg was evaluated in NSG mice bearing BT-474 subcutaneous tumors treated daily for 21 consecutive days (Fig 2).
Treatment with neratinib was well tolerated at both doses, producing minimal body weight change. The 50mg/kg dose regimen resulted in complete tumor regression in all mice. Treatment at the lower dosage level resulted in ~50% tumor growth inhibition, suggesting a dose response. This data provides clear evidence that the BT-474 model has maintained its HER2 overexpression and dependency.
BT-474 – New Life to a Powerful Preclinical Tool
Given the continual need for new targeted breast cancer treatments, from small molecules and antibodies to cell-based therapies, it is our responsibility to optimize the unique cell lines that we have available for drug discovery research.
The use of models like BT-474 that can bridge the gap between preclinical model and cancer patient is crucial. Optimization of our BT-474 breast cancer model in NSG mice has been a significant advancement of our validated breast tumor models, and we are continuing to evaluate other HER2 targeted therapies, such as Herceptin, in the BT-474 model.
Please contact us to speak with our scientists about how BT-474 or one of our other models can be used for your next oncology study.


